WHO’s GTH-B holds second training course for biomanufacturing experts from Low- and Middle-Income Countries (LMICs)
- Regdate2022-10-31 14:35
- Hit4,888
WHO’s GTH-B holds second training course for biomanufacturing experts from Low- and Middle-Income Countries (LMICs)
세계보건기구 글로벌 바이오 인력양성 허브, 중저소득국 백신 생산인력 2차교육 실시
PRESS RELEASE
OCT 31, 2022
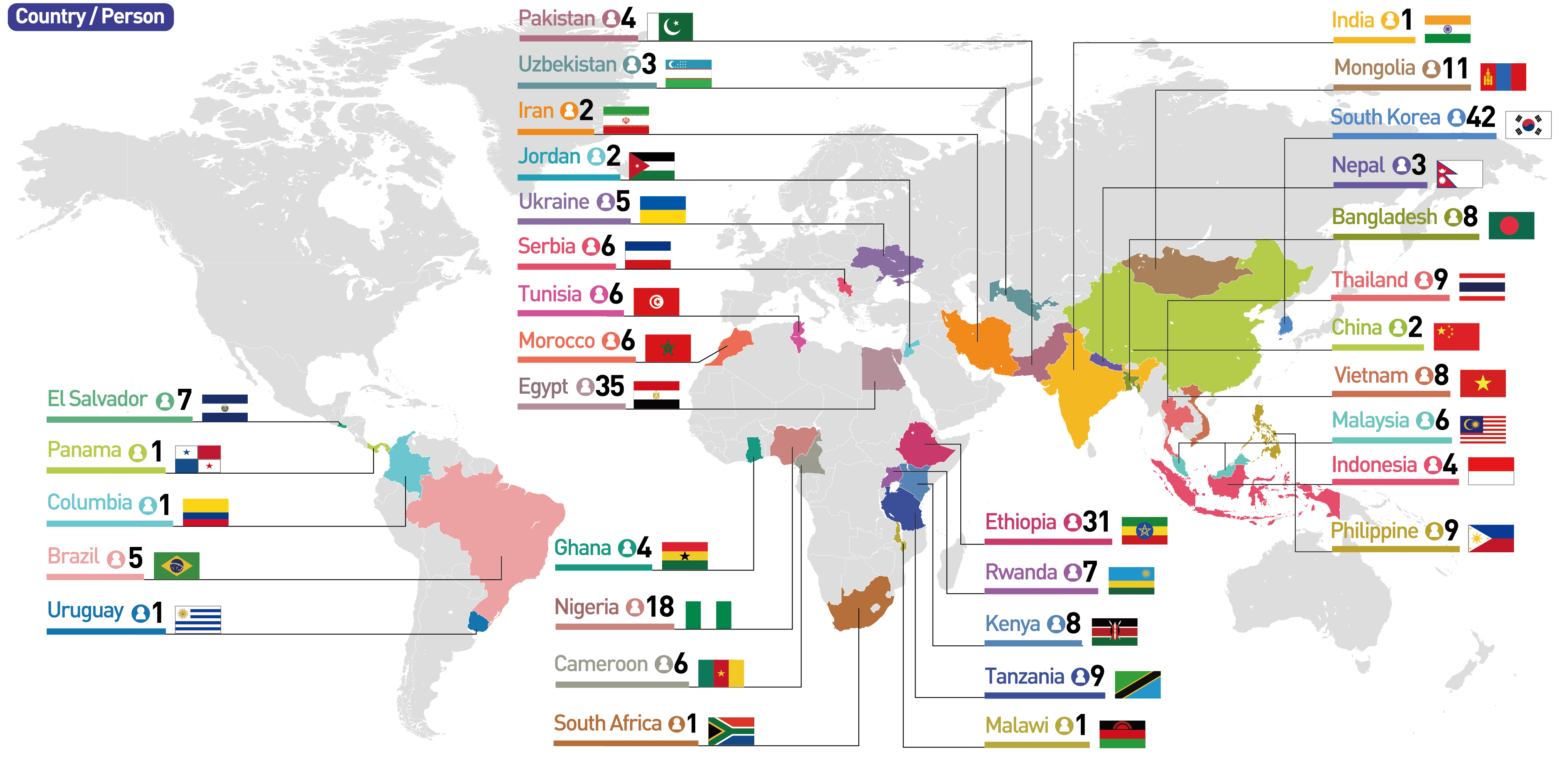
Starting with the Welcome Session on the 31st of October (Monday), the Ministry of Health and Welfare launched its Introductory Training Course for Standard Practice (GxP) in Biomanufacturing, together with 230 biomanufacturing experts from 33 Low- and Middle-Income Countries (LMICs) and 42 trainees from Korea.
This program, which will take place at the Seoul National University’s Siheung Campus for the next 3 weeks, is the second training course organized by Korea since its designation as a Global Training Hub for Biomanufacturing (GTH-B) by WHO.
Korea successfully organized and hosted the Introductory Course for Biologics Development and Manufacturing last July, which was offered for 2 weeks to 138 trainees from 25 nations.
Identical to the first program, the second training course will be offering certificates of completion jointly signed by the Minister of Health and Welfare of Korea and the Director General of the International Vaccine Institute to the trainees that successfully complete the curriculum.
The training program in July provided an introductory, theory-based course on the overall lifecycle of biologics, including the development, manufacturing, and approval of vaccines and medicine.
However, the current program is focused on biosafety, which is required for safe and effective production of biologics, as well as quality management, including GMP (Good Manufacturing Practice) and GLP (Good Laboratory Practice).
Through this program, trainees will learn about detailed certification criteria applicable to the overall manufacturing process, including the facilities, raw materials, production, and packaging, for the production of vaccines and biologics.
Moreover, this program will offer a theoretical course on quality management as well as other activities, including group case studies, convention programs with local vaccine and biologics manufacturers, and visits to their production facilities, over a total of 120 hours over three weeks.
A Ministry of Health and Welfare official explained that the introductory course on biologics development and manufacturing, offered last July, confirmed the passionate enthusiasm and high satisfaction of the trainees from LMICs, and reaffirmed Korea’s aspiration to provide a highly satisfactory program that meets the expectations of the participants with quality lectures and a variety of activities offered as part of the current course on the certification criteria for quality management.
One of the trainees who attended program as a researcher in July cited the opportunity to build a human network with experts in various fields hailing from many nations and the content of the training as the main reasons for satisfaction, while noting that “it’s not easy to meet vaccine experts from such a wide range of countries even as a current practitioner in the field.”
Everest Okeakpu who is a project manager of Biovaccines Nigeria Limited, stated that “Africa is not equipped with the infrastructure to manufacture and store vaccines, making it difficult to vaccinate people”, while expressing a strong intention to “apply and integrate what was learned during the training to existing plans in Africa, which currently is working toward building an mRNA Hub.
The current global biomanufacturing training was promoted via the websites of overseas diplomatic missions of LMICs and foreign embassies in Korea, the International Vaccine Institute (IVI), and the World Health Organization (WHO) since mid-July and applications were received through the IVI homepage.
The trainees are mainly composed of current employees of public and private companies as well as government offices and public institutions specializing in vaccines and biologics within the LMICs. A total of 230 final trainees were selected after discussion with the WHO.
After completing the 3-week quality management (GxP) training, the attendees will be assigned to the vaccine manufacturing sites of relevant companies or government institutions back home, and are expected to take on major roles in the building of manufacturing bases, research·education, and the supply of vaccines.
in Biomanufacturing (10.31.~11.18.)>
The entrance ceremony to mark the start of this education was held at 10 am. on October 31 (Monday) at the Seoul National University Siheung Campus Yeonsu-dong & Convention Center.
Tedros Adhanom Gebreyesus, secretary-general of the World Health Organization(WHO), who cooperated in education program after designating Korea as a "Global training hub for biomanufacturing" said in a video message, the hub’s role is to “support LMICs to produce vaccines and other biologics,” and “WHO has established the first global training hub for biomanufacturing in the Republic of Korea.” regarding the nation’s superior biomanufacturing infrastructures and resources. And, Director General Ghebreyesus emphasized WHO will continue to cooperate with public and private organizations around the world, including Korea, to successfully foster vaccine production personnel, which “requires long-term collaboration between the public and private sectors.”
During his congratulatory remarks during the ceremony, the Head of the Global Vaccine Hub Task Force Kim Hyun-Joon of the Ministry of Health and Welfare highlighted the importance of talent development, stating that “Korea, as a nation that operates a global biomanufacturing training hub, is providing a solid foundation to fight against the pandemic by fostering manufacturing experts in vaccines and medicine from around the world.”
Director General Jerome Kim of the International Vaccine Institute commented, “the quality management training course supplements and builds on the introductory course offered back in July to enhance the biomanufacturing capacity that is much needed in LMICs” and added, “the IVI will support the local production of vaccines and biologics in these nations by offering high-quality training on quality management (GxP) by actively leveraging the outstanding bio-resources and training infrastructure of Korea.
Several international organizations engaged in the fight against future infectious diseases, including the Coalition for Epidemic Preparedness Innovations (CEPI), and the Bill & Melinda Gates Foundation (BMGF), also took part in the training program by offering virtual special lectures.
Korea will continue to build on a robust and sound training program for WHO’s Global Training Hub for Biomanufacturing and become the undisputable leader in biomanufacturing talent development by enhancing cooperation with international organizations, including the WHO, and by offering more practical training.
The 2022 introductory courses on the manufacturing process of vaccines and medicine and quality management organized by the International Vaccine Institute will become more diversified and more comprehensive in scope to help improve the capacity and satisfaction of trainees.
Cooperation with international organizations, including the WHO, CEPI, ADB, IDB, and BMGF, will be further expanded to develop and provide training programs that meet the demands of the world as a whole and each specific region.
In particular, the courses will add more practical curriculum elements “Hands-on training” to the program by leveraging the outstanding infrastructure of Korea to help support improvements in the vaccine and medicine manufacturing capacity of the trainees.
// For inquiries, contact Media Relations, Ministry of Health and Welfare
044-202-2047 or fairytale@korea.kr
http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&page=1&CONT_SEQ=373424
-
 (10.31)WHO’s GTH-B holds second training course for biomanufacturing experts from Low- and Middle-Income Countries (LMICs)_vf.hwp
( 1.79MB / Download 389. / Preview 270. )
Download
미리보기/음성듣기
(10.31)WHO’s GTH-B holds second training course for biomanufacturing experts from Low- and Middle-Income Countries (LMICs)_vf.hwp
( 1.79MB / Download 389. / Preview 270. )
Download
미리보기/음성듣기
-
 (10.31)WHO’s GTH-B holds second training course for biomanufacturing experts from Low- and Middle-Income Countries (LMICs)_vf.pdf
( 244.14KB / Download 380. / Preview 264. )
Download
미리보기/음성듣기
(10.31)WHO’s GTH-B holds second training course for biomanufacturing experts from Low- and Middle-Income Countries (LMICs)_vf.pdf
( 244.14KB / Download 380. / Preview 264. )
Download
미리보기/음성듣기